Statera Biopharma
Statera Biopharma’s foundation is based on restoring immune health through the leveraging of complex, pleiotropic beneficial effects. At Statera, we embrace the idea that beneficial and safe drugs act as natural analogs in a paracrine fashion, as well as a trans-activator to resolve disease and elicit homeostasis.
Statera is going to change the way people think about immunotherapy. Specifically, we are going to develop our pipeline with these guiding principles:
Restoration & Balance
Comprehensive & Complex
Safety & Foresight
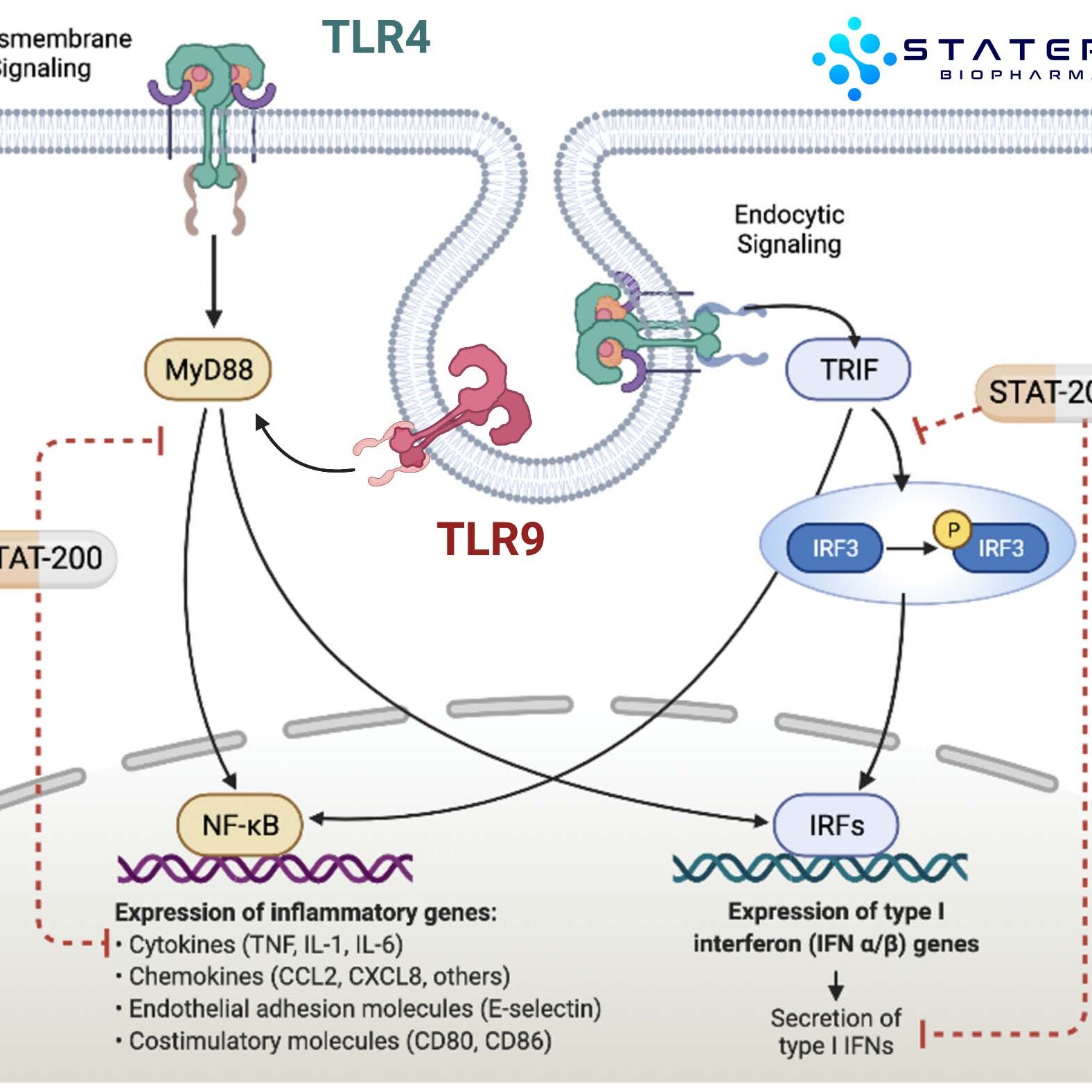
AIMS (ADVANCED IMMUNOMODULATING MULTI-COMPONENT SYSTEM)
Protective immunity and resolution to health (homeostasis) is orchestrated in parallel by multiple cell subsets, surface receptors, soluble factors and intracellular pathways. One cell, molecule or linear pathway is rarely the sole agent of immune dysfunction (disease). Years of research with naltrexone and met-enkephalin confirmed their anti-cancer and anti-inflammatory properties are a result of immune modulation through multiple cell types, receptors, and pathways. Leveraging that experience, STATERA developed its core development platform, AIMS.
AIMS allows us:
- to rapidly assess how novel compositions impact multiple pharmacokinetic-pharmacodynamic relationships, cellular/molecular selectivity, and overall therapeutic potency;
- to discover with potentially greater accuracy, through methodical property analysis, targets with the improved activity and levels of therapeutic benefit not achieved by other published immunotherapies that use a single target approach;
- to formulate a new class of immune-restorative drugs that harmonize the immune orchestra (by targeting complex pathways and engaging toll-like receptors);
- to comprehensively engage the immune system such that our compositions treat disease, and help resolve inflammation, restoring balance, rather than a one-dimension approach of blunting or exaggerating a single factor.
AIMS Programs
Featured News
Statera Biopharma, Inc. to Participate in the A.G.P. Biotech and Specialty Pharma Conference
Statera Biopharma Announces CEO Mike Handley to Speak During Virtual B & T Cell-Mediated Autoimmune Disease Drug Development Summit
Events
10/6-10/7 2021: Statera Biopharma Announces CEO Mike Handley to Speak During Virtual B & T Cell-Mediated Autoimmune Disease Drug Development Summit
Statera Biopharma, Inc. (Nasdaq: STAB), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, today announced that Michael K. Handley, President and Chief Executive Officer, has been invited to participate as an expert speaker during the 2nd Annual B & T Cell-Mediated Autoimmune Disease Drug Development Summit. The virtual conference is taking place October 6-7, 2021.
The details of Mr. Handley’s presentation are as follows:
| Event: | B & T Cell-Mediated Autoimmune Disease Drug Development Summit |
| Date: | Thursday, October 7, 2021 |
| Session: | Novel Approaches to Disrupting B & T Cell Collaborations |
| Time: | 8:00 a.m. ET |
| Registration: | https://b-and-t-cell-for-autoimmune.com/register/ |
9-30-2021: Statera Biopharma, Inc. to Participate in the Cantor Virtual Global Healthcare Conference
Statera Biopharma, Inc. (Nasdaq: STAB), a leading biopharmaceutical company creating next-generation immune therapies that focus on immune restoration and homeostasis, will participate in the 2021 Cantor Virtual Global Healthcare Conference taking place September 27-30, 2001. Michael K. Handley, President and Chief Executive Officer of Statera, will participate in a virtual fireside chat on Thursday, September 30, 2021.
Registration:

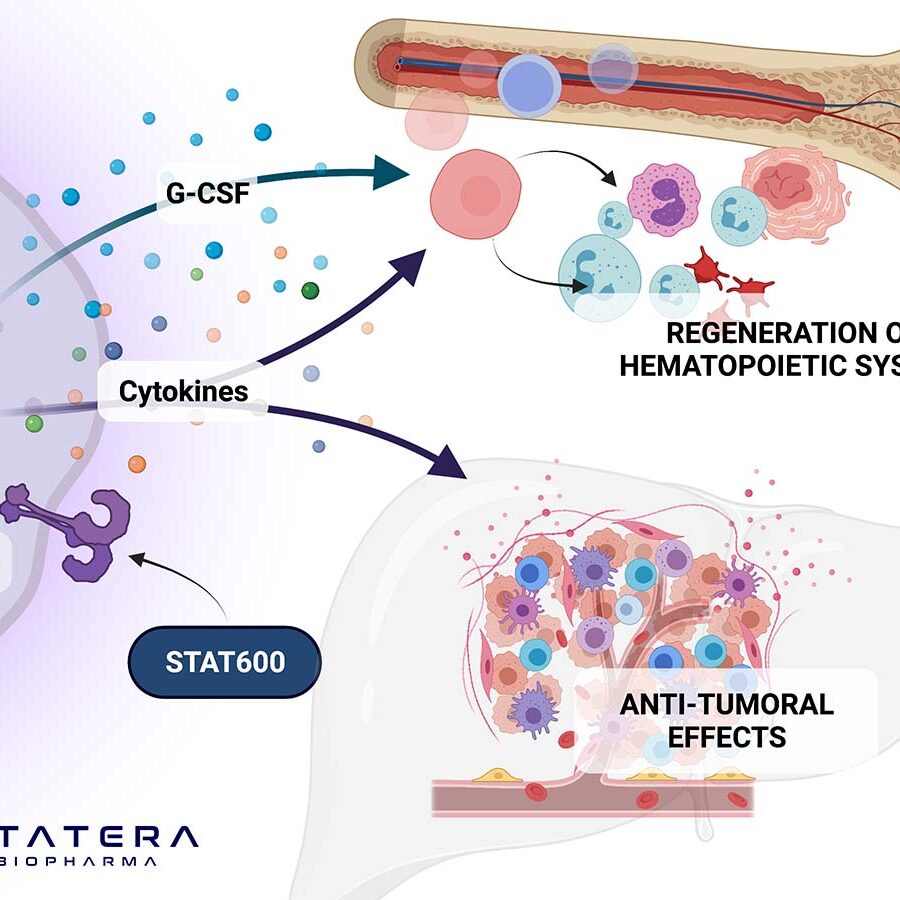
STAT-205 is expected to potential treat acute and post-acute COVID infections by modulating immune system function and by decreasing elevated inflammatory responses associated with SARS-CoV-2 viral infections, making it a potential candidate for the treatment of Acute and Post-Acute COVID 19.
With the FDA having cleared the Investigational New Drug (IND) application, Statera expects to begin clinical trials in the third quarter of 2021 to evaluate the pharmacokinetics and biomarkers of STAT-205. Preliminary in vitro data have shown to slow or halt the progression of SARS-CoV-2 in human lung cells, the virus that causes COVID-19.

Statera completed a successful end of Phase 2 meeting with the U.S. Food and Drug Administration (FDA) regarding a clinical development plan for a Phase 3 clinical trial evaluating STAT-201 in pediatric Crohn’s patients. Cytocom expects to begin enrolling patients by year-end 2021.

Statera’s STAT-401a is an injectable pentapeptide that is targeted for development as an adjunct to the standard of care to extend the duration of disease remission in patients with pancreatic cancer. With recent FDA feedback, Type C meeting, regarding the clinical development program and establishing an advisory panel of oncology experts, we are planning to initiate a clinical trial in the first half of 2022.

Statera is also finalizing protocols for a study evaluating STAT-205 in patients with post-acute COVID-19 syndrome (PACS). Also known as “long haulers,” these patients represent a high unmet medical need as roughly 30% of all COVID-19 infections develop into long-haul syndrome. Statera plans to conduct the trial under the existing cleared IND and expects to begin enrolling patients by year-end 2021.