Subsidiaries & Partners
Genome Protection, Inc.
Genome Protection, Inc. (GPI), is a partially owned subsidiary making significant inroads in the longevity space
We are a longevity biotech company

Our founders and scientists have dedicated their lives to studying the genome and its role in aging and cancer. We believe that aging is a disease and thus can be treated. Our goal is to develop such treatments to significantly prolong healthy lifespan and protect against the many diseases of aging.
Genome protection is our primary area of expertise
We have come a long way in our quest
Areas of Focus
Retrobiome management
Immunostimulation
Retrobiome vaccination
ImQuest Life Sciences
ImQuest Life Sciences, Inc., is a contract research and development company focused specifically on cancer, inflammation and infectious disease treatments. ImQuest Pharmaceuticals was merged into Statera’s existing drug development operations, and ImQuest Biosceinces, the contract research organization (CRO), operates as wholly-owned, revenue-generating subsidiary of Statera Biopharma, inc..
Customized Drug Discovery and Development Services
 ImQuest BioSciences is a preclinical CRO that provides expert services to evaluate the potential of new and novel pharmaceutical products for the treatment and prevention of viruses, bacteria, cancer and inflammatory diseases. These preclinical research services are part of our ImQuestSUCCESS Platform and include compound screening to define compound efficacy and drug target validation to define the mechanism of action and toxicity of pharmaceutical products.
ImQuest BioSciences is a preclinical CRO that provides expert services to evaluate the potential of new and novel pharmaceutical products for the treatment and prevention of viruses, bacteria, cancer and inflammatory diseases. These preclinical research services are part of our ImQuestSUCCESS Platform and include compound screening to define compound efficacy and drug target validation to define the mechanism of action and toxicity of pharmaceutical products.
We specialize in services for the development of small molecules, natural products, biologics, antimicrobial peptides, therapeutic antibodies and vaccines.
We are committed to earning our client’s trust and building long term relationships through collaboration, unwavering commitment to quality science and consistent and effective communication. Our team has decades of experience in preclinical contract research. As such, we understand that each product and each client is unique and we strive to provide effective solutions.
Areas of Focus
- Virology
- Microbiology
- Oncology
- In vitro Toxicology
- Pharmaceutical Properties and Characterization
- Microbicides and STI Prevention
- Supportive Technical Services
- Consulting Services
Panacela Labs
Panacela Labs, is a majority-owned subsidiary developing Mobilan, based on non-replicating adenovirus vector delivery system encoding TLR5 and corresponding ligand
 Panacela Labs Inc. maintains a focus on oncology and orphan drug develo pment, some drugs of which are on the stage of clinical studies and is developing Mobilan, an innovative anticancer drug product in collaboration with the Roswell Park Cancer Institute (Buffalo, USA) and Cleveland Clinic Foundation (Cleveland, USA).
Panacela Labs Inc. maintains a focus on oncology and orphan drug develo pment, some drugs of which are on the stage of clinical studies and is developing Mobilan, an innovative anticancer drug product in collaboration with the Roswell Park Cancer Institute (Buffalo, USA) and Cleveland Clinic Foundation (Cleveland, USA).
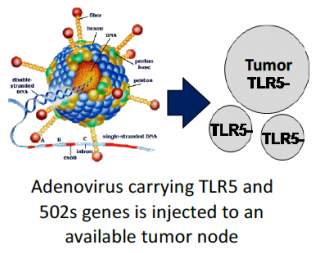
Mobilan’s action includes transduction of tumor cells with bicistronic recombinant non-replicating adenovirus which manages expression of human Toll-like receptor of 5 type (TLR5) and its specific ligand-agonist, 502s, pharmacologically optimized analogue of bacterial protein flagellin. The derived constitutive autocrine stimulation of signaling pathway TLR5 results in response of innate immunity and subsequent development of adaptive anti-tumor immune reactions.
The proprietary technology allows to convert any available tumor node to the vaccine activating innate and further acquired immunity which may be used for treatment of various cancer neoplasms.
Areas of Focus
Prostate Cancer
Incuron, LLC
Incuron, LLC. is a royalty partner with Statera focused on the development of innovative medicines for the treatment of cancer and auto-immune diseases, based on Curaxins – a novel class of chemical structures
 Incuron’s clinical lead curaxin CBL0137, demonstrated significant anti-cancer activity in a number of preclinical tumor models, including colon, pancreatic, prostate and breast adenocarcinomas, melanoma, kidney cancer, glioblastoma, hematological malignancies, and several pediatric malignancies, including neuroblastoma. It showed efficacy both as a monotherapy and in combination with standard of care and various targeted therapies. In animal studies, significant inhibition of tumor growth, and in some cases complete tumor eradication, was observed at non-toxic concentrations of. CBL0137 is currently being tested in several clinical studies against solid and hematological malignancies.
Incuron’s clinical lead curaxin CBL0137, demonstrated significant anti-cancer activity in a number of preclinical tumor models, including colon, pancreatic, prostate and breast adenocarcinomas, melanoma, kidney cancer, glioblastoma, hematological malignancies, and several pediatric malignancies, including neuroblastoma. It showed efficacy both as a monotherapy and in combination with standard of care and various targeted therapies. In animal studies, significant inhibition of tumor growth, and in some cases complete tumor eradication, was observed at non-toxic concentrations of. CBL0137 is currently being tested in several clinical studies against solid and hematological malignancies.
Areas of Focus
Solid Tumors, IV administration, USA
Solid Tumors, Oral administration, RUS
Hematological Malignancies


